The overall goal of the Translational Optics Imaging and Spectroscopy lab is to harness light-tissue interaction, in order to develop novel optical instrumentation and imaging methods/algorithms, for the functional imaging of tissue physiology. Optical techniques are inherently sensitive to endogenous biomarkers of tissue health, such as blood flow, tissue oxygen saturation (StO2) and hemoglobin concentration. Direct measurement of oxygen delivery (i.e., from blood flow) and oxygen consumption (i.e., from oxy-/deoxy-hemoglobin) also permits the computation of tissue metabolism, which can shed light on the normal (or abnormal) functioning of tissue. Our research aims to measure/image these tissue biomarkers with novel and custom optical instrments for a variety of clinical and pre-clinical applications, including brain imaging, cancer, and muscle. Herein, we highlight a few major research themes.
Bedside non-invasive optical monitoring of healthy and injured brain
Optical medical devices offer a portable and non-ionizing solution to directly measure cerebral physiology non-invasively at the bedside. This is in contast to conventional imaging modalities (e.g., MRI/CT) that are expensive, require the use of a radiological suite, and are used only a few times during a patient's hospital stay. With optics, monitoring of cerebral tissues is realized via portable fiber-optics connected to an optical instrument (see figure) placed at the bedside of the patient/subject. Our custom devices typically measure cerebral blood flow, cerebral blood oxygenation, and hence cerebral metabolic rate of oxygen (CMRO2); but are also equipped to record systemic physiology such as blood pressure, heart rate etc.
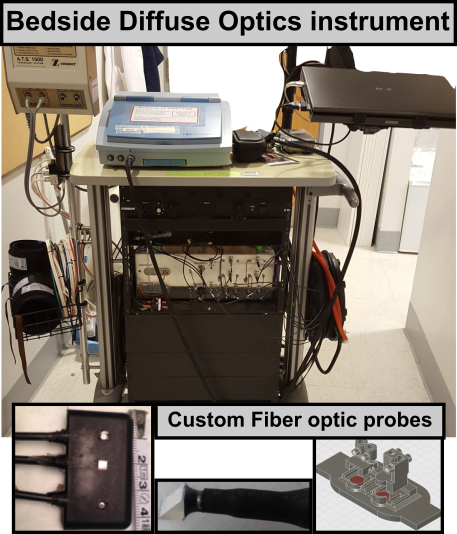
See Diffuse Optics for more discussion on the different technologies and implemention of diffuse optical instruments.
Current research in this area is focused on the development of novel instrumenation strategies, methods and algorithms, in the following application areas :
-
Critical care monitoring & ischemic stroke recovery:
During critical care of brain injuries, neurologists typically aim to optimize cerebral blood flow and oxygenation in the affected regions so as to contain and/or treat injured brain tissues. Reserach in this area is focused on the development of faster, more compat, and patient-friendly opto-electronic instruments with real-time computation and display of tissue blood flow and oxygen saturation. Continuous measurements of cerebral hemodynamics may also be used to develop data-driven models that predict secondary injuries, deterioration or recovery. -
Quantiatification of Cerebral Autoregulation:
Cerebral Autoregulation (CVAR) refers to the ability/mechanism that, in the normal brain, maintains relatively constant CBF (i.e., regulates CBF), despite fluctuations in the blood pressure. In the injured brain, CVAR is thought to be impaired, and the extent of impairment is a predictor of injury severity. Research in this area is focussed on developing algorithms for estimating an index/biomarker of autoregulation, using data from novel clinical diffuse optical instruments.
Imaging and modeling cerbrovascular diseases
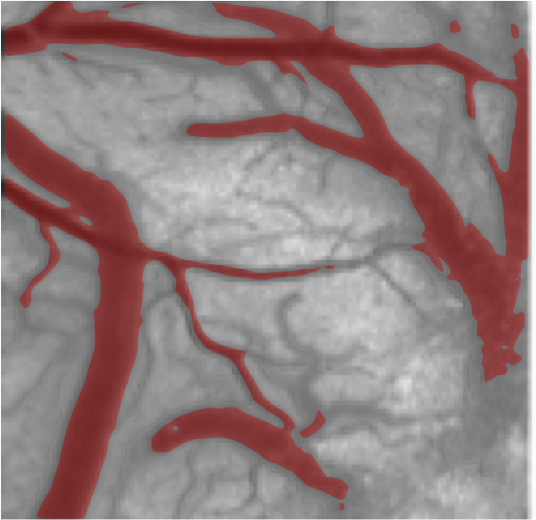 Measurable physiological parameters such as cerebral blood flow and cerebral oxygenation are effective markers of brain health, and the ability to image and map cerebral vasculature (and changes thereof), is important for the pathophysiological modeling of cerebrovascular diseases such as ischemic stroke, imaging/guiding of cerebrovascular surgeries, and development/testing of clinically relevant treatment strategies. In contrast to macroscopic techniques such as Magnetic Resonance Imaging, optical methods offer the ability to image individual blood vessels, and deliver images of cerebral physiology with high spatial and temporal resolution.
Measurable physiological parameters such as cerebral blood flow and cerebral oxygenation are effective markers of brain health, and the ability to image and map cerebral vasculature (and changes thereof), is important for the pathophysiological modeling of cerebrovascular diseases such as ischemic stroke, imaging/guiding of cerebrovascular surgeries, and development/testing of clinically relevant treatment strategies. In contrast to macroscopic techniques such as Magnetic Resonance Imaging, optical methods offer the ability to image individual blood vessels, and deliver images of cerebral physiology with high spatial and temporal resolution.
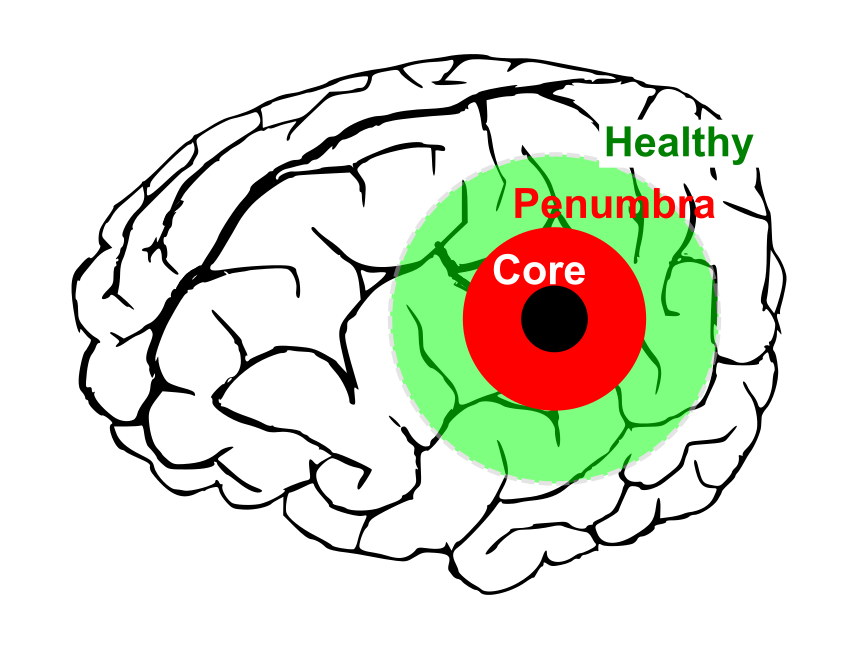 For example, a focal cerebral ischemia (i.e., stroke, see figure), is often modeled as having an ischemic core (wherein blood flow drops below 20% of baseline), surrounded by a penumbra (wherein blood flow is usually 25-30% of baseline) and healthy tissue. While the core consists of dead tissue, the penumbra has electrical silent neurons, but is potentially salvageable. However, this reduction in penumbral blood flow limits the tissue’s ability to supply the extra metabolic demand that results from secondary effects such as peri-infarct depolarizations (PIDs) and peri-infarct flow transients (PIFTs), which lead to worsening of ischemic conditions and increase in size of the core infarct. Stroke treatment strategies target the restoration of penumbral blood flow, and overall improvement of brain oxygenation.
For example, a focal cerebral ischemia (i.e., stroke, see figure), is often modeled as having an ischemic core (wherein blood flow drops below 20% of baseline), surrounded by a penumbra (wherein blood flow is usually 25-30% of baseline) and healthy tissue. While the core consists of dead tissue, the penumbra has electrical silent neurons, but is potentially salvageable. However, this reduction in penumbral blood flow limits the tissue’s ability to supply the extra metabolic demand that results from secondary effects such as peri-infarct depolarizations (PIDs) and peri-infarct flow transients (PIFTs), which lead to worsening of ischemic conditions and increase in size of the core infarct. Stroke treatment strategies target the restoration of penumbral blood flow, and overall improvement of brain oxygenation.
Our research in this area is focused on the development of new optical imaging instruments to image the progression of stroke at high spatial and temporal resolutions, specifically targeting observational limits of current technology. In particular, we utilize methods based on Laser Speckle Contrast Imaging, and Intrinsic Optical imaging to measure cerebral blood flow and blood oxygenation respectively. We are also interested in studying the effects of simple treatment strategies that offer neuroprotective effects and prevent (or in some cases reverse) the progression of stroke. Finally, a long-term goal is to clinically translate these findings/strategies to in vivo use in humans.
See also Intraopeartive Imaging, and Laser Speckle Contrast Imaging.
Modeling light propagation in tissue
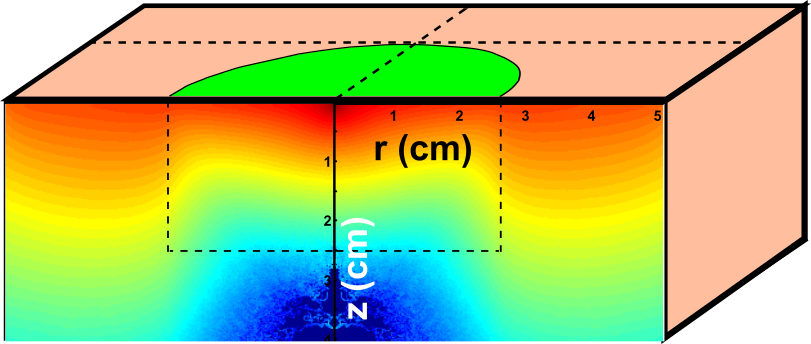
An important part of our research concerns the exploration of how light interacts with tissue using analytical and computational tools. Analytical models (e.g., the photon diffusion equation) have clinically-useful closed form mathematical solutions, but only for simple (i.e., homogeneous) tissue geometries and conditions. Computational methods (e.g., Monte Carlo simulations, Finite Element Modeling) can help address some of these limitations, and can simultate light propagation in realistic and heterogenous tissue. Research in this area is focussed on development of efficient computer simulations of light tissue interaction, in order to understand clinical measurements/images of tissue blood flow (e.g., with Laser Speckle, or Diffuse Correlation Spectroscopy), blood oxygenation (e.g., with Diffuse Optical Spectroscopy) and/or tissue fluorescence. In particular, our goal is to use these simulations to address assumptions in analytical methods, and/or drive the development of new biomedical optics technology.